Using Human Genetic Information to Find Effective and Affordable Therapies
 Many people suffer from rare and/or complex diseases with no known treatment options. Despite an understanding of the molecular basis of almost six thousand diseases, we only have about 500 approved therapies. Our hope is to drive a shift in the drug development paradigm in order to efficiently identify drug repurposing candidates that are safe, affordable, and most importantly effective, to help improve the lives of these patients.
Many people suffer from rare and/or complex diseases with no known treatment options. Despite an understanding of the molecular basis of almost six thousand diseases, we only have about 500 approved therapies. Our hope is to drive a shift in the drug development paradigm in order to efficiently identify drug repurposing candidates that are safe, affordable, and most importantly effective, to help improve the lives of these patients.
The goal of the Accelerating Drug Discovery and Repurposing Incubator (ADDRI) is to discover new uses for FDA-approved drugs based on an understanding of diseases driven by human genetics. The genetic data we use is from BioVU, Vanderbilt’s deidentified human DNA biobank. The repurposing program is overseen by Jill Pulley, Executive Director of the Vanderbilt Institute for Clinical and Translational Research (VICTR), and led by a team of scientific project managers. The incubator includes a multidisciplinary think tank of experts in various disciplines, including basic scientists, clinical researchers and business and legal experts.
We start by identifying potential genetic variants of interest based on Phenome Wide Association Studies (PheWAS) performed using our deidentified medical records link to BioVU DNA samples. If there are approved drugs that target a specific protein of interest, we leverage the PheWAS results to discover novel gene-disease associations. The team then executes in-depth evidence reviews to determine the plausibility and viability of novel drug-indication pairs. The final step in project initiation is to design experimental approaches to validate new indications.
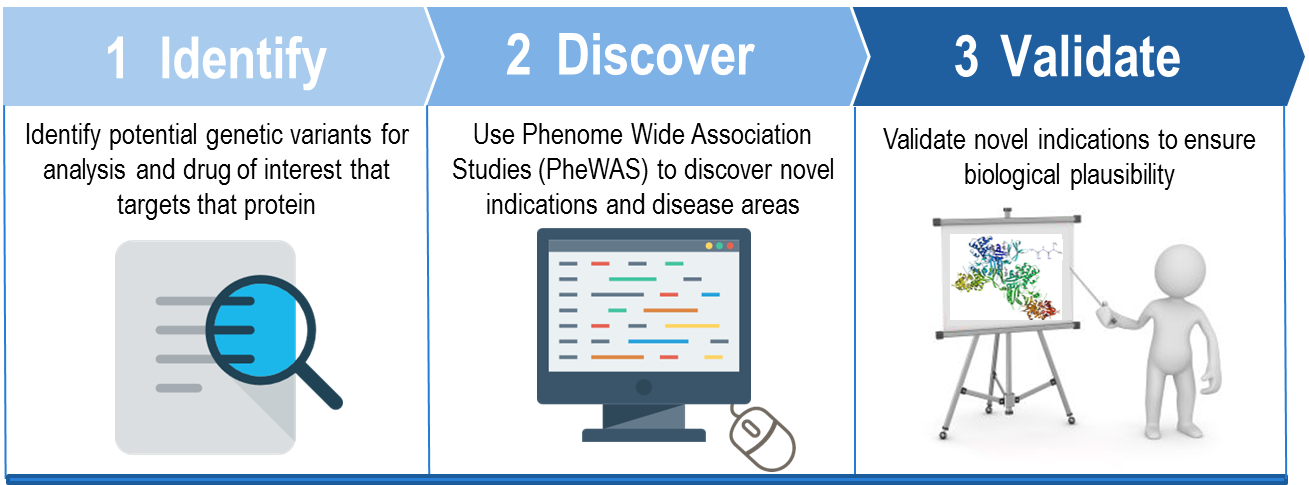
Capitalizing on our intimate knowledge of chemistry, biochemistry, and protein structure, the Center for Knowledge Management (CKM) was asked to collaborate with the Drug Repurposing team as they develop new project concepts. CKM is primarily involved in the three early phases of analysis depicted to the right.
CKM contributions include:
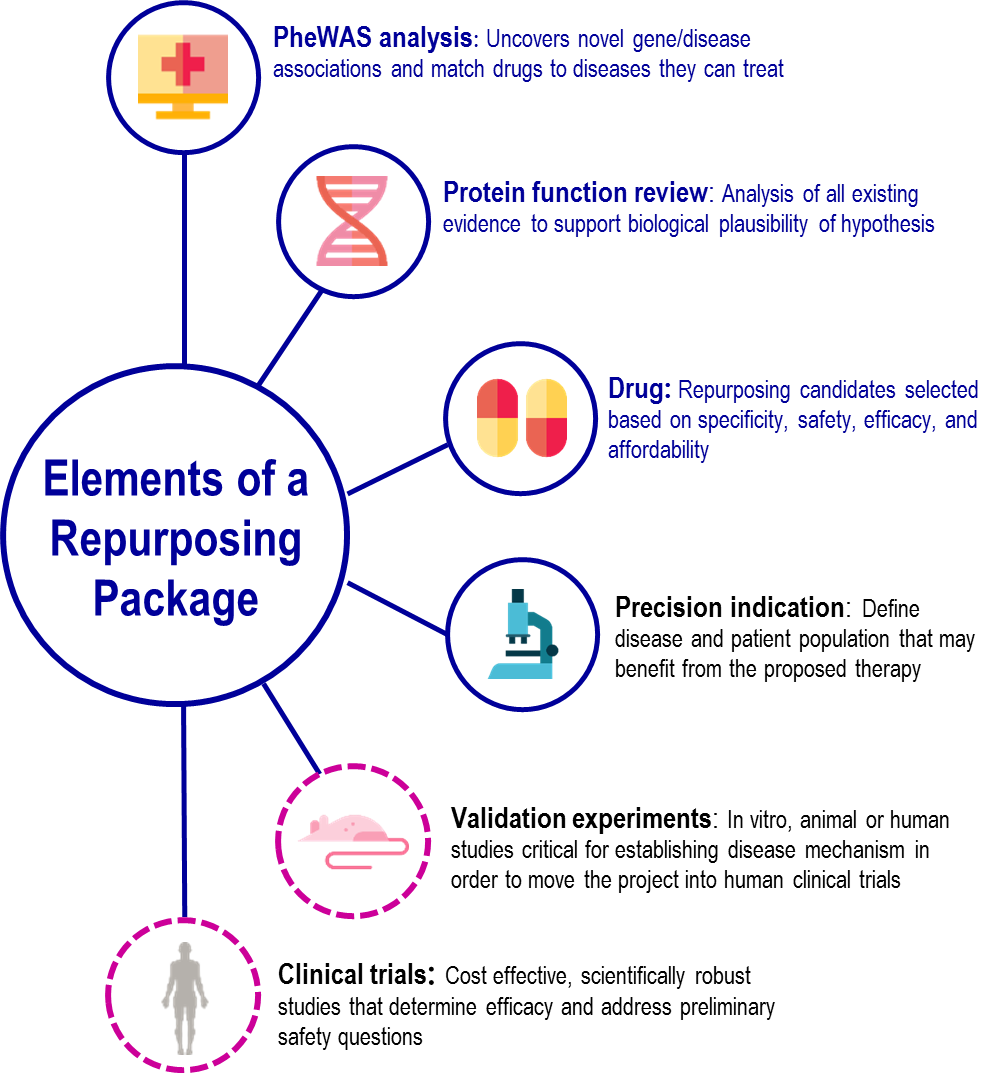
- Identifying variant and wild type alleles for specific mutations
- Ensuring the frequency of variant alleles is consistent with reported allele frequencies in our population(s) of study
- Reviewing PheWAS results in the context of how each genetic mutation may affect the expression, structure, function, and/or pathway interactions of our protein drug target of interest
- Analyzing all current evidence and prediction algorithm output to support the biological plausibility of hypotheses and new drug indications
- Collaborating to find already approved drugs that target our protein of interest with the desired pharmacological effect and safety profile
Subsequent to validation, the repurposing team aims to develop a commercially viable package for licensing to support larger scale registration trials and development activities.